Abstract
Introduction
TP53 mutations have been associated with poor prognosis across the spectrum of hematologic malignancies. In myeloproliferative neoplasms, TP53 mutations have been frequently associated with blastic phase implicating them in disease progression1. However, previous studies have confirmed that TP53 mutations only rarely occur in PMF showing incidences of 4% (n=104) 2, 2% (n=50) 3, and 1% (n=182) 4. Given the rare occurrence of TP53 mutations in PMF, the clinicopathologic features of TP53mut PMF are not well characterized. In one of the largest studies to date of PMF with NGS data (including TP53), we sought to further delineate the molecular and clinical features of TP53mut PMF in 131 patients with disease.
Methods
This study was approved by the Institutional Review Board. PMF cases from our institution for which NGS data was available were identified. NGS data was recorded for all cases from CAP/CLIA certified testing performed during the course of routine clinical care. NGS panels included Genoptix 21-gene panel, Custom 31 gene TrueSeq Myeloid panel, and standard 54-gene TruSeq Illumina Myeloid panels. All tests reported mutation variants with an allele frequency of 5% or more. FISH results (mostly FISH for MDS panel, including del(5q), del(7q), del(17p), del(20), and trisomy 8) were retrieved. Cytogenetics by conventional karyotyping was performed at LabCorp, and these results were manually curated and recorded. The clinical ambulatory reports, pathology reports, and pathology slides were reviewed to confirm or revise the diagnosis as appropriate. Diagnoses were rendered following the WHO 2008 classification for hematopoietic malignancies. Two-way Fisher's Exact Test was used for statistical analysis.
Results
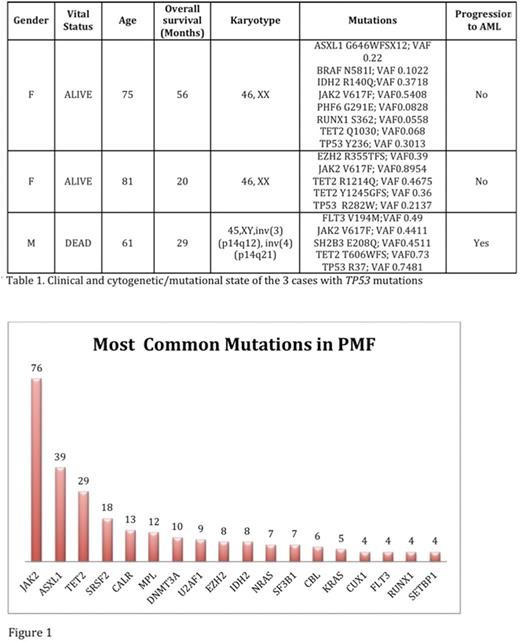
There were 131 PMF cases with NGS identified. The average age was 69 years. There were 73 males (56%) and 58 females (44%). Myeloid associated gene mutations were identified in 116/131 cases (89%). The most commonly mutated genes identified by NGS were: JAK2 (76), ASXL1 (39), TET2 (29), SRSF2 (18), CALR (13), MPL (12), DNMT3A (10) (Figure 1). TP53 mutations were detected in 3 cases (2.3%): TP53 Y236; variant allele frequency (VAF): 0.3013; TP53 R282W; VAF: 0.2137, and TP53 R196*; VAF 0.7481. The average number of mutations in TP53mut PMF is 5.7/case, which is much higher than the number identified in TP53 wild type PMF patients 2.2/case (p= 0.0001). One of the three TP53mut patients (33%) had complex cytogenetics versus 3 of 87 TP53 wild type PMF cases (3.4%) (p=0.13). The other two TP53 mut cases had normal cytogenetics. The TP53mut patient with complex cytogenetics underwent transformation to acute myeloid leukemia (33%) versus 5/128 (3.9%) in TP53 wild type PMF (p=0.13). Average overall survival was 35 months for TP53mut PMF versus 50.3 months for TP53 wild type PMF (p= 0.65). The genomic profile of TP53mut PMF is provided below in Table 1. Interestingly, all 3 TP53mut cases harbored both JAK2 and TET2 mutations. Also of note, B-cell monoclonal lymphocytosis was seen in 66% of TP53mut PMF.
Conclusions
Our study, one of the largest to date (n=131), confirms that TP53 mutations are rare in PMF. TP53mut PMF are more likely to shows higher comutation rates, complex cytogenetics, progression to AML, and poorer median survival. TET2 mutation was found in all TP53 mut cases. In murine models of JAK2 V617F positive myeloproliferative neoplasms, the loss of TET2 resulted in progression and loss of p53 proteinin frank AML 5. Thus, our findings support the notion of p53 as a biomarker of aggressiveness in PMF and shed light on the pathogenesis of disease. Association of clonal B-lymphocytosis with TP53mut PMF may warrant further investigation of underlying immune response in these cases.
References
1. Yogarajah, M. Tefferi, A. Mayo Clin Proc2017, 92 (7), 1118-1128.
2. Raza, S. et al. Am J Hematol2012, 87 (2), 204-6.
3. Greaves, W. O. et al. Leuk Lymphoma2013, 54 (7), 1552.
4. Tefferi, A. et al. Blood Advances 1:105-11 .
5. Rampal, R.; et al. Proc Natl Acad Sci U S A2014, 111 (50), E5401-10.
Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau. Sallman: Celgene: Research Funding. Padron: Incyte: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.